A Quick Note
I’d like to note that this is an oversimplified explanation of HLA, which means there are a lot of generalizations here. Exceptions exist to these concepts. However, if you are new to HLA this will hopefully be an easy (but somewhat comprehensive) introduction.
Jump to the Key Takeaways at the bottom of the page for a quick overview.
The Short Answer
What is HLA? Anyone who works in HLA has a ready answer to this question because it gets asked a lot when you work in the field.
Here’s my short answer:
HLA molecules are proteins located on almost all of the cells in your body. They help the immune system identify self from non-self which makes them extremely important when matching donors and recipients for transplant compatibility.
So what’s the longer answer? It’s a bit more complicated, but let’s start at the beginning. This article introduces a lot of concepts that will be explored in more detail in other posts.
The Long Answer: What is HLA and what does it do?
Macroscopically, HLA molecules help the immune system recognize self from non-self and this role is highlighted when looking at transplantation. Microscopically, HLA molecules present pieces to the immune system that help determine if an infection is present. HLA molecules also have some connection to disease associations. But first, let’s talk about the vocabulary of HLA.
HLA: The Vocabulary
HLA stands for Human Leukocyte Antigen. To break that down:
Human = our human body
Leukocyte = This is a word for the white blood cells (WBCs) that exist in your blood and throughout your body. These white cells contribute to your immune system. The words ‘leukocytes’ and ‘white blood cells’ can be used interchangeably.
Antigen = The word ‘antigen’ in immunology refers to a molecule that your immune system can potentially make antibodies to. Size matters. Essentially, an antigen is big enough for your immune system to recognize it as a problem. In the case of HLA, the antigens are glycoproteins.
So HLA molecules are human leukocyte antigens. Which is deceptive. While these molecules are present on white blood cells (and play a huge role in the immune system), they are also located on other cells in your body.
Now that you know what the acronym stands for, no one ever calls them ‘human leukocyte antigens’ unless they’re 1) not used to working with them or 2) clarifying for someone. You can call them HLA molecules or HLA antigens.
HLA molecules can be typed in a transplant laboratory or specialty HLA laboratory using a simple cheek swab, mouthwash sample, or a blood sample. Depending on the testing, it could take 48 hours or a week to perform an HLA typing.
If your HLA molecules have been typed, you can refer to the HLA specificities that make up your HLA typing. For example, HLA-DRB1*01 is a type of HLA specificity and is distinct from the HLA-DRB1*04 specificity. HLA nomenclature is the system for naming these HLA specificities.
Regardless of which specificities you have, HLA molecules are glycoproteins that are located on almost all of the cells in your body. This makes them valuable in determining what is self and what is non-self.
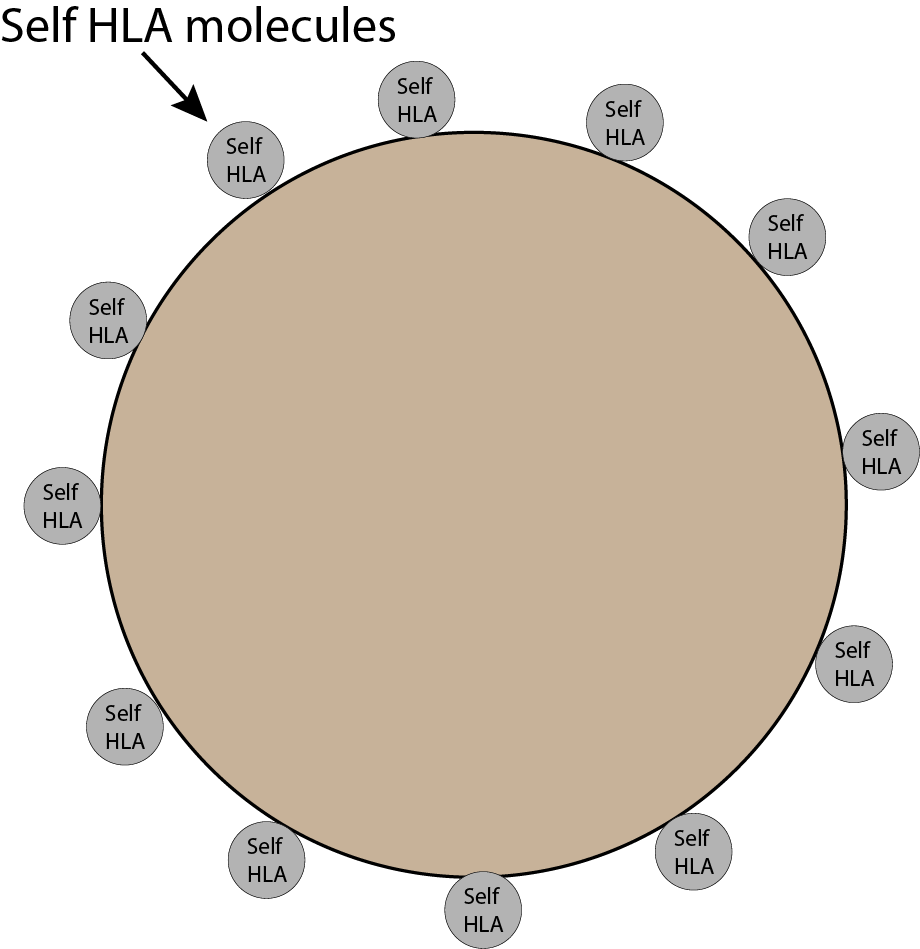
The Big Picture
HLA’s Macroscopic Role: Self vs Non-self
HLA molecules help the immune system identify what is self and what is non-self. Your HLA type is specific to you. That makes it ‘self’ to your immune system.
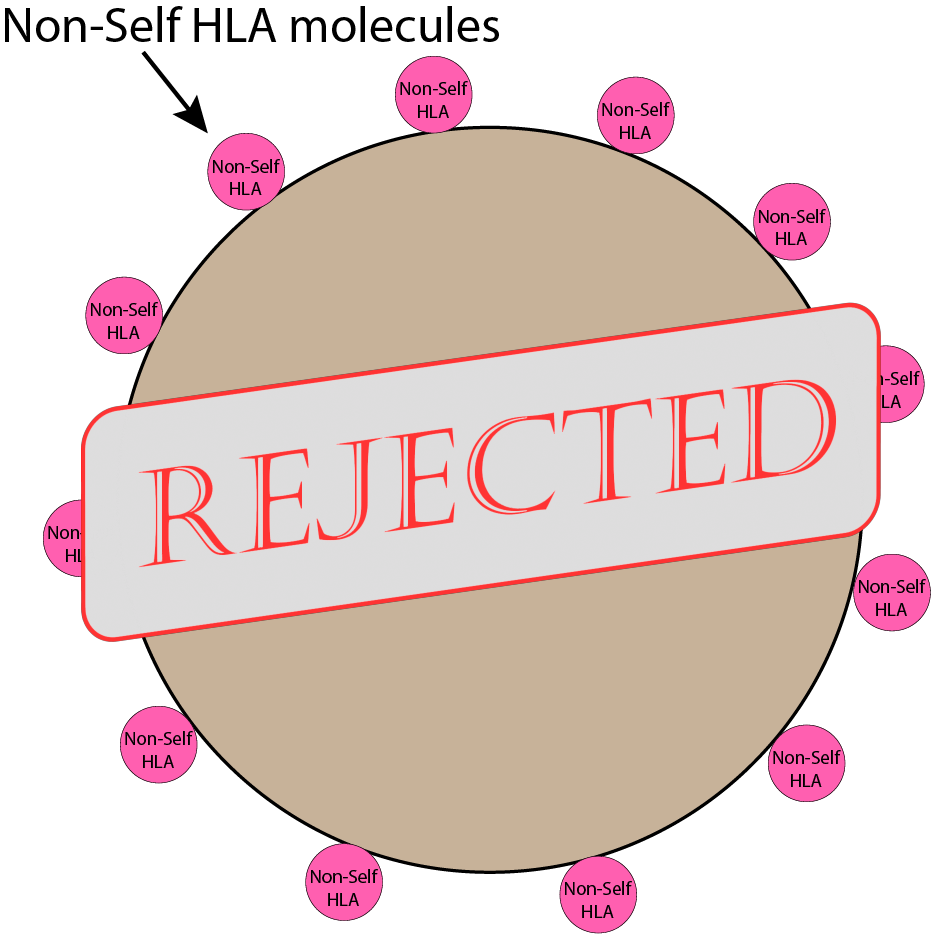
So what is non-self? Pretty much everything else.
- If it’s a different HLA type, it’s non-self. This includes:
- transplanted organs (contains the donor’s HLA type)
- some transfused blood products (contains the donor’s HLA type)
- If it doesn’t have ANY HLA molecules, it’s non-self. This includes:
- Bacteria
- Viruses
- any other pathogenic substance large enough to elicit an immune response that lacks the necessary HLA molecules.
- If your own cells don’t have HLA molecules present (this occurs with some cancers), these cells are also killed. No HLA molecules means the immune system does not identify the cell as self and will kill it. This is one way our body naturally prevents cancer cells from spreading.
Just imagine your body, your skin, your cells, your organs, your blood vessels. They all have little self-HLA molecules on them.
Now imagine, you get a paper cut and bacteria gets into your body. Bacteria do NOT have your HLA molecules on them; in fact, they have no HLA molecules at all (humans only, sorry bacteria). The immune system notices the bacteria is without your self-HLA (and notices a few other things, like the molecular patterns on their cell surfaces) = destroys the bacteria. No self-HLA hall pass, no entry.
Everyone must have the correct hall pass to be in your body. This is the general concept of ‘self’ vs ‘non-self’.


Keeping in mind that this is an oversimplified version, this is generally the easiest drawing to show molecules on a cell. There are many other molecules on the cell surface that are completely unrelated to HLA but for now we’re only focused on the HLA molecules.
The number of HLA molecules on a cell differ based on a lot of factors. Some cells display more HLA molecules on their surface, some display less. Other cells display only certain varieties of HLA molecules (as in HLA Class I vs HLA Class II) but that’s too much detail to get into here.
The cells in your body are going about their business. Lung cells are keeping you breathing, kidney cells are helping you pee, liver cells are detoxifying your blood, your WBCs (the white blood cells that help make up your immune system) are patrolling your body to make sure ‘self’ is all it sees. Any sign of trouble and your immune system will round up the troops to attack.
Immune System Surveillance and HLA Molecules
So we know that ‘self’ means that it belongs in your body; ‘Non-self’ means that it does not belong in your body.
But who’s doing the surveillance? Your immune system (those WBCs). Each cell in the immune system has a role in how it surveils the body. T cells, for example, are excellent at HLA surveillance.
T cells are patrolling the body, keeping an eye on HLA molecules, something like security officers in a building. Not all immune cells are looking at the HLA molecules, but T cells love this gig.
Passing cells show their HLA molecules as a way to prove they belong in the body, like an ID badge to get you past security. What those HLA molecules are holding is also important (more on this in a minute).
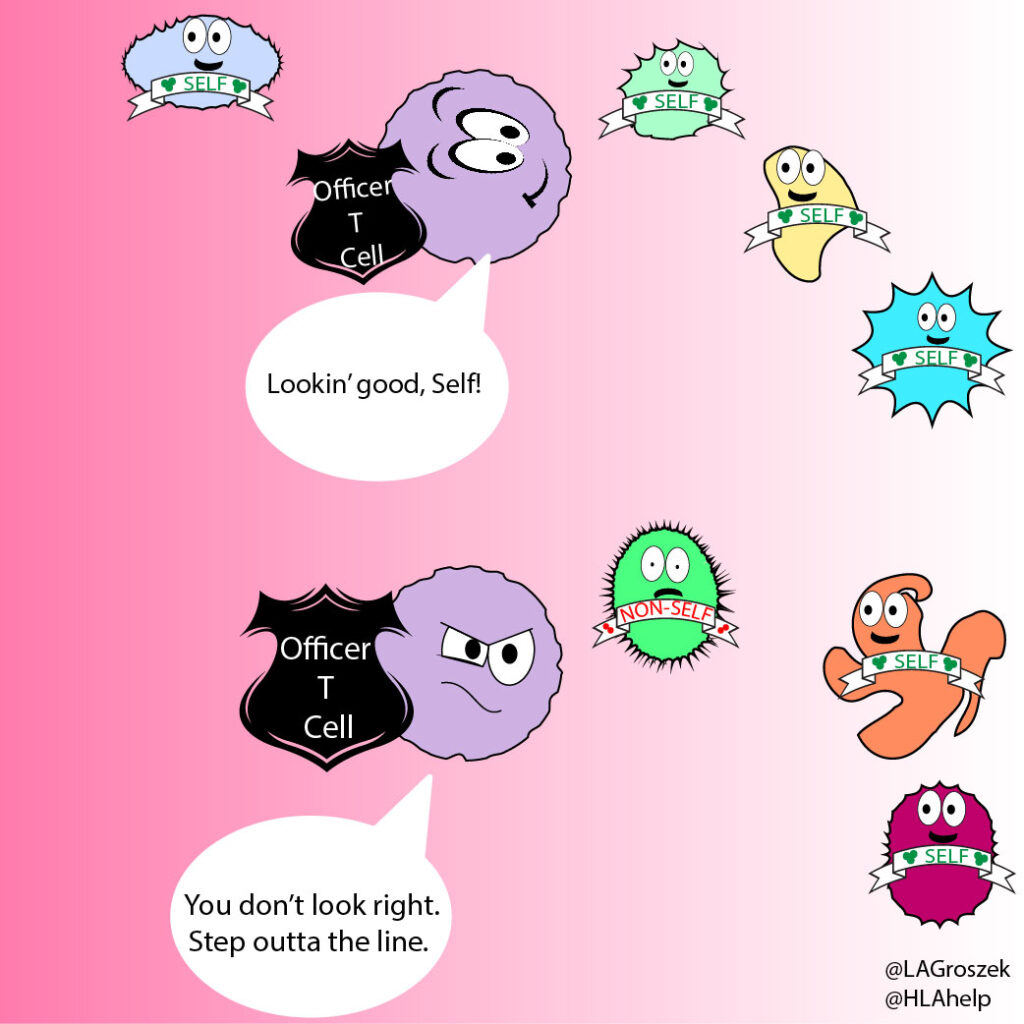
The T cells frisk other cells continuously by looking for self-HLA molecules. If the cell doesn’t pass the test, the T cell can activate the immune system to remove, inactivate, or otherwise kill the invader.
If there are a lot of invaders, the signal sent out by immune cells will amplify, effectively calling in other immune cells to help clear out the danger. Cells communicate by sending out chemical signals (cytokines) that cause other immune cells to migrate towards the designated area. Like…say…directly to a transplanted organ.
Why match HLA molecules for a transplant?
As your immune system is looking around and checking for self-HLA molecules, some HLA differences are glaringly obvious while others are more subtle.
Look at these two pictures. Imagine the flags are the cell’s HLA type, where the Australian flag is self. Can you spot the non-self flag in each picture? You can use the slider to reveal the invader.

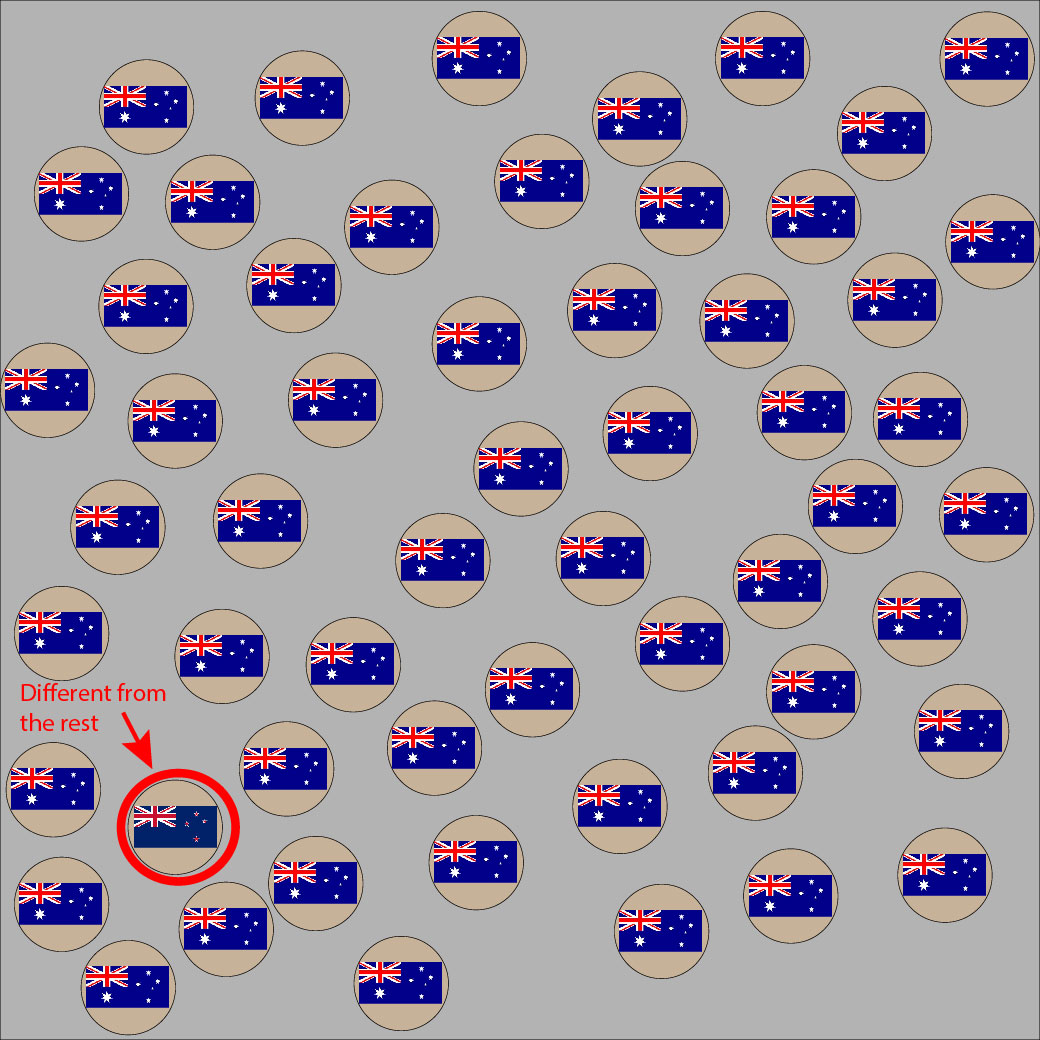

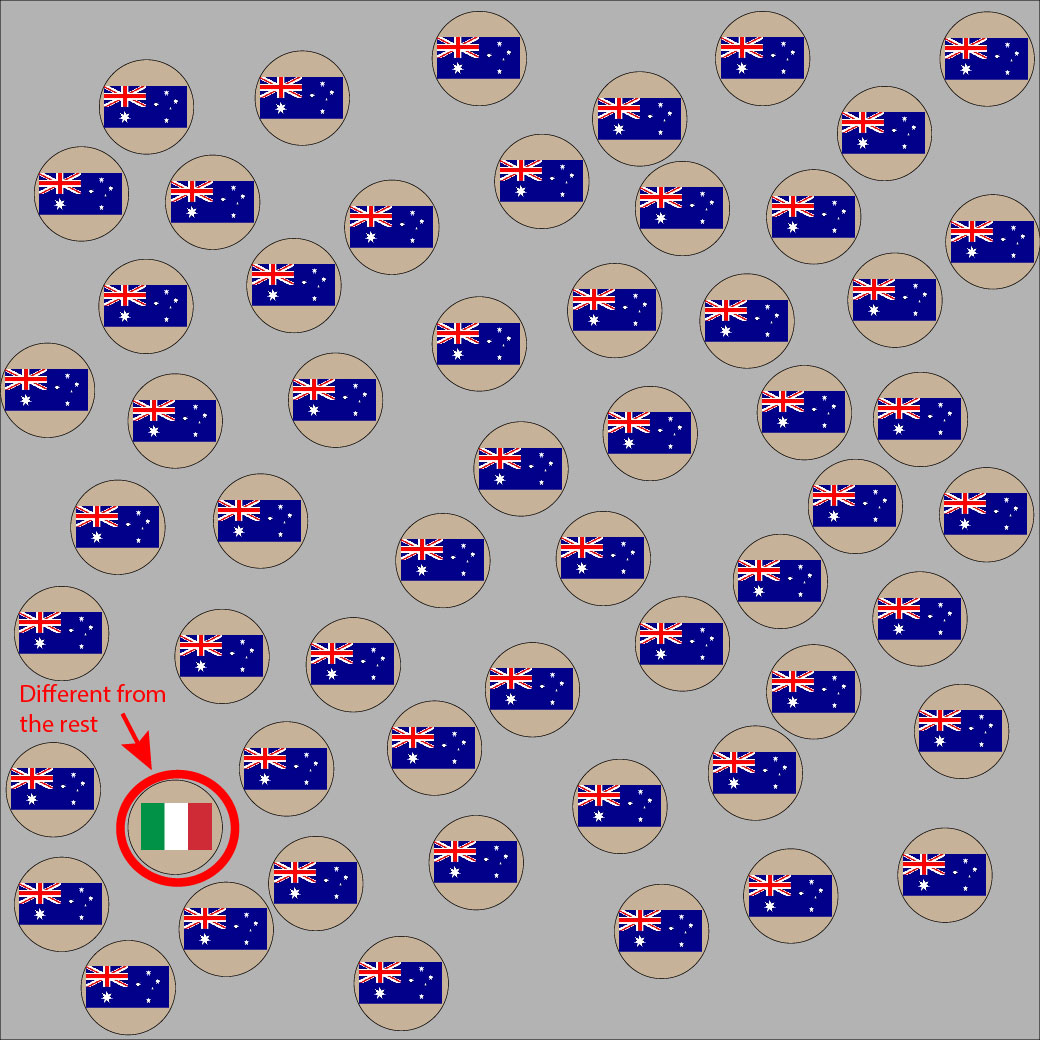
If your pattern game is on point, your eyes were drawn to the second outlier almost immediately because the green/white/red colors in the Italian flag are so very different from its surroundings.
If a donor organ’s HLA molecules are too obviously different from the host, the immune system can spot the difference fast. If the immune system sees the transplanted organ as ‘non-self’, cells will begin attacking the invading organ, ultimately leading to organ rejection. Thanks to modern medicine, things like immunosuppressant drugs prevent the immune system from recognizing or acting on these differences.
This is why it’s handy to have an HLA match that is close in type. Because HLA is genetically inherited, you get your HLA type from your parents. A match from a sibling or parent has better a chance of success because you share the same (or similar) ‘self’ HLA molecules.
Again, an oversimplification reminder. There are additional details and qualities to factor in when matching donors and recipients, but the goal right now is general understanding of HLA’s role. There are also additional differences between how HLA is matched for bone marrow compared to solid organs (like the lungs and the heart) that will be discussed another time.
The Smaller Picture
HLA’s Microscopic Role: Presenting Antigenic Peptides
HLA molecules help the immune system identify self from non-self.
What else do HLA molecules do? A cell with HLA molecules can use these molecules to present small antigenic peptides to the immune system. This gives T cells a taste of what’s hanging out in the body as they go about their surveillance because they are the one’s looking at the presentation.
In essence, these presented antigenic peptides are amino acid strands that are large enough to trigger an immune response, but small enough to fit into “the pocket” of an HLA molecule. (HLA molecules have pockets! so cute, right?) The pocket is referred to, more officially, as a peptide binding groove.


Where do these antigenic peptides come from? They come from within the cell that’s presenting them. The cell is constantly building and breaking down contents within itself. Some of these broken down pieces get popped into the HLA pocket and presented to T cells.
The pieces being presented are often self-peptides and the T cell is trained (from its baby T cell stages) to recognize these self-peptides as ‘self’. This means T cells are looking at the HLA molecule itself and what that molecule is holding in its pocket. This is another way that T cells can recognize self from non-self (by what the pocket is holding).
What happens when an HLA molecule is presenting non-self peptides?
Macrophages (another type of WBC), for example, can eat up invaders (like infectious bacteria) in their surroundings. Macrophages like to eat stuff. If there are bacteria near the macrophage, he’ll eat them up and then break down the bacterial cell into pieces that can be presented in the HLA pocket for the T cells to check out.
The T cell can recognize that the macrophage’s HLA molecule is self but that the peptide it’s holding is non-self. This means the macrophage has found something that doesn’t belong in the body. Once activated in this handshake, the T cell uses chemical signaling (cytokines) that communicates to the immune system that invaders are present. This is a normal function of the immune system to identify infections.
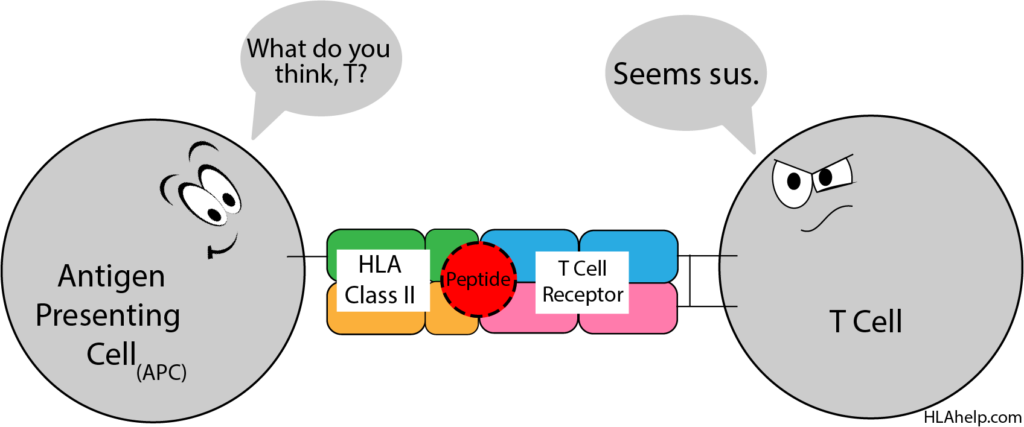
Macrophages are known as antigen presenting cells (APCs); only a few distinct cell types in the body act as APCs.
Most cells (not APCs) are passively living their lives and doing the job that the body has assigned them to do (like lung cells involved in breathing). They have no interest in galivanting off to find new and interesting things to show T cells. They show off whatever is already present in their cell (hopefully self-antigenic peptides) by popping a peptide in their HLA pockets and calling it a day. If a T cell comes by and checks it out, they’ve done their duty.
APCs, on the other hand, are actively looking for things in their surroundings to grab and show T cells. It’s a core part of their job in the body.
This handshake between the T cell and the APC involves multiple different connections between cells before activation is confirmed. This is known as a T cell/APC interaction; the types of APC and T cell determine the details of the interaction and the action that follows.
So what is the T cell learning when checking out the HLA molecule of its neighboring cell?
- The HLA molecule tells the T cell whether or not the cell belongs in the body.
- The antigenic peptide sitting in the pocket of the HLA molecule tells the T cell whether or not that cell has found an invader that needs to be destroyed.

Disease Associations
Autoimmunity and T Cells
HLA molecules play a role in training T cells.
In order for T cells to understand what is self and what is non-self, they are quizzed as youngsters in the Thymus. They also need to be trained in proper etiquette and response when meeting HLA molecules. We don’t want T cells overreacting or underreacting.
HLA molecules play a role here in making sure the T cell shows future HLA molecules the proper respect. This means: no hugging so tightly that T cells will grab anything that moves (causing immune system activation of things it shouldn’t be attacking), but no ignoring HLA molecules either. Like Goldilocks and the Three Bears, this connection between T cell and HLA molecule has to be just right.
A T cell that is not properly trained might be overly attracted to and excited about everything, including presentation of self-peptides. Because this T cell doesn’t distinguish self from non-self, it can trigger the immune system to attack self, causing autoimmune diseases; this is when the person’s immune system starts attacking their own body because it mistakenly considers a part of the body to be an invader when it’s not. In other words, the T cell could love the peptide literally to the death.


Looking at the opposite response, a T cell that ignores HLA molecules and attaches to nothing will allow invaders to spread disease within the body.
Both options are not good.
We need T cells that are trained to properly recognize the HLA molecule, paying proper attention to the HLA molecule as well as the peptide presentation. HLA molecules play a part in this training during the early stages of T cell development.

Autoimmunity and HLA Structure
Molecular Mimicry
Because HLA molecules play an extensive role in our immune system, HLA molecules are thought to affect how our body relates to particular diseases.
HLA molecules are extremely polymorphic, meaning there are a HUGE variety of different HLA specificities (molecule types) out there. Some differences between molecules are miniscule, as we noted with the Australian and New Zealand flags. Some differences are obvious, like the differences between the Australian and Italian flags. These specificities differ in their structure which influences the types of peptides that they can hold and present to T cells.
In molecular mimicry, an invader gets into your body and happens to look like a part of your body that is self. The immune system mistakes self for being part of the invasion and begins attacking in what becomes an autoimmune response.
Molecular Mimicry Scenario: Let’s say a golden retriever invades your body. A passing cell eats the invader, chops it up, presents it on your HLA molecule. The T cell sees the presentation and marks golden retrievers for death (as someone who loves golden retrievers, keep in mind this is only an example). The immune system gets to work destroying golden retrievers.
What happens if there’s a part of your body that looks like a labrador retriever? It’s not quite a golden retriever, but it’s close. (Those ears, am I right? <3)

This isn’t a matter of poor training on T cell’s part, it’s a matter of molecular mimicry. Your body saw an invader (the golden retriever) and attacked it, as it should. Unfortunately, the invading golden was also presented in a way that it looks A LOT like the labrador that hangs out in your joints. Now your immune system is after the labrador as well, creating an autoimmune scenario that results in your experiencing joint pain and inflammation.

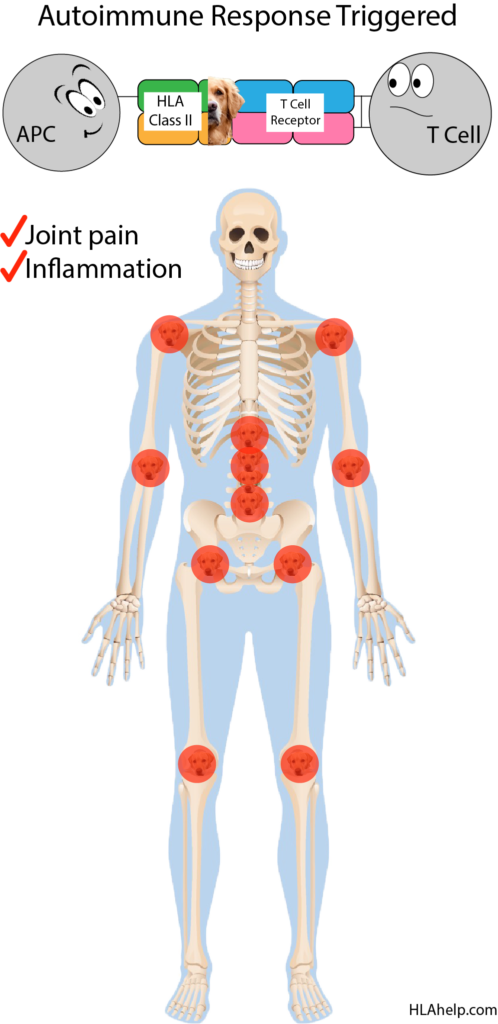
The point is, your immune system has trouble identifying self from non-self if the two look really similar.
However, different HLA specificities present peptides differently.
Another HLA molecule may not show the golden retriever the same way. This means that, with a different HLA specificity (maybe HLA-DRB1*04 instead of HLA-DRB1*01), the way your immune system sees the golden retriever may look different.
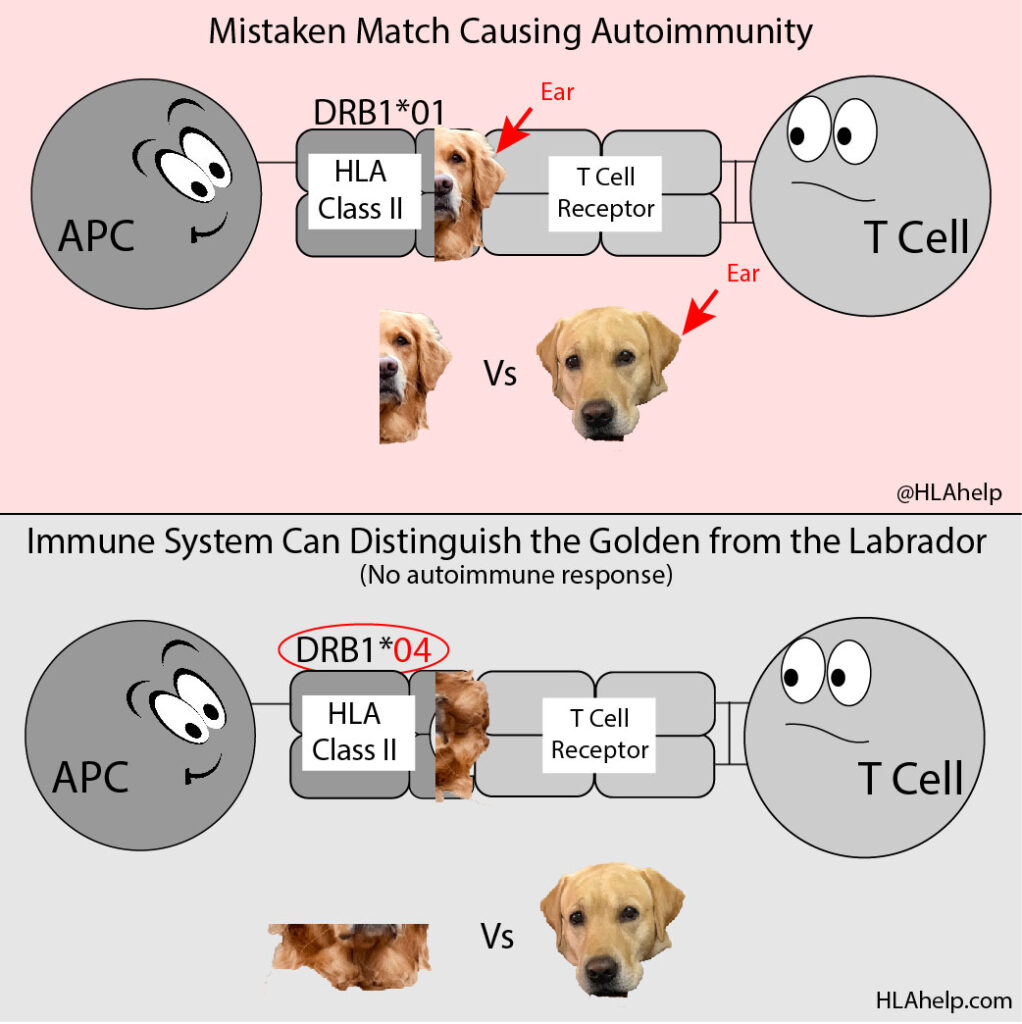
The HLA-DRB1*01 and the HLA-DRB1*04 are different types of HLA Class II molecule. Their structures are different at the molecular level and present different parts of the invader based on what their pocket is capable of holding.
Where the DRB1*01 showed the ear and instigated an autoimmune response by accident, the DRB1*04 is structured slightly differently and showed the neck of the golden retriever instead.
If DRB1*04 presents the neck, all of a sudden it doesn’t look so much like the labrador. You’re spared the autoimmune response because the immune system can attack the golden retriever by zeroing in on its neck instead of its ear. Because the necks of the golden and labrador look distinctly different, the labrador is safe (and so are your joints).
This is also why the HLA system has evolved to become so polymorphic and why you have several different types of HLA molecule within your body: more variety is better protection.
Protections
While certain HLA specificities might make us susceptible to some diseases, they are capable of protecting us as well. In other words, your HLA type might make you more susceptible to disease X but that same type provides stronger protection against disease Y.
Each HLA specificity can have advantages and disadvantages that affect how our immune system reacts to any particular infection or disease.
Key Takeaways
- HLA molecules:
- are glycoproteins on the surface of most cells in the body.
- play a key role in determining what the immune system sees as ‘self’ and ‘non-self’.
- present peptides in their peptide binding groove (the pocket!).
- are constantly being surveilled by T cells to determine that the HLA molecule and the peptide in its pocket are both ‘self’.
- train T cells to help prevent autoimmune reactions.
- Non-self HLA molecules or non-self peptides trigger an immune response.
- Organ and bone marrow transplantation use HLA typing (among other qualities) to match donors and recipients.
- Molecular mimicry can potentially cause an autoimmune response.
- HLA specificities differ in molecular structure, which affects the peptides that can bind in the pocket.
- The variety of HLA specificities increase protection against potential invaders.
Core Concepts
- HLA Nomenclature (the system of naming HLA specificities)
- HLA Peptide Binding Groove (The Pocket!)
- HLA Matching for Organ and Bone Marrow Transplantation
- HLA Peptide Presentation
- T cell Development
- T Cell/APC Interaction
- Disease Associations
Credits
Photo Credits
Thanks to:
grapixmania on vecteezy.com for the human body skeleton.
Photo by Yogiraj Banerji on Unsplash for the labrador retriever.
Photo by Emil Priver on Unsplash for the golden retriever.

